The International Society of Sports Nutrition published in 2017 a position stand (see reference below) on the safety and efficacy of creatine supplementation in the context of exercise, sports and medicine.
Creatine supplementation, one of the most popular and studied nutritional supplements, has in fact been shown to be effective in improving athletic performance (especially in high intensity exercise) and inducing relevant training adaptations. The consequent increase in intramuscular creatine (and phosphocreatine) reserves facilitates the rapid re-synthesis of ATP, the so-called energy “currency” of the body, which is essential for almost every reaction in our body. Thus, the increased availability of creatine in the cell through supplementation contributes to improve performance because it increases the energy availability in order to exercise (i.e. muscle contraction) as well as a whole range of other muscle cell related reactions. Creatine supplementation can in fact enhance strength production, muscle work, accelerate recovery and help preventing injury.
Additionally, creatine supplementation appears to be highly safe and effective not only in athletes but also in non-athletes (such as the so-called exercise enthusiasts), as well as in various clinical populations. In fact, several studies (see ISSN article, reference below) point to benefits of creatine supplementation in various populations and clinical settings, such as:
– Accelerating injury rehabilitation (because it attenuates muscle atrophy);
– Protection of neuronal injuries (spinal and cerebral);
– Mitigation of debilitating consequences in people with congenital syndromes of creatine synthesis deficiency;
– Attenuating the progression of neurodegenerative diseases (e.g. Huntington’s disease, disease, Parkinson’s disease, mitochondrial diseases, amyotrophic lateral sclerosis);
– Prevention and / or improvement of bioenergetics in patients with myocardial ischemia or stroke victims;
– Improving metabolic and functional indicators associated with aging;
– Possible benefit during pregnancy for optimal growth, development and health of the fetus.
In conclusion, creatine does indeed appear to be a safe and beneficial nutritional supplement for a wide range of populations and ages. Indeed, this is a supplement that actually works!
Take creatine and power to you!
Nuno Correia
References:
Kreider, R.B. et al., 2017. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. Journal of the International Society of Sports Nutrition, 14(1), p.18. Available at: http://jissn.biomedcentral.com/articles/10.1186/s12970-017-0173-z.
In order to maintain physical function, recover better from injury, maintain lean mass and stay healthier overall, older individuals need a higher protein intake than younger individuals. Higher protein intake contributes for attenuating inflammatory and catabolic processes, as well as ameliorating the decreased efficiency in protein metabolism associated with age.
According to the recommendations advanced by the PROT-AGE Study Group (2013) (reference below), older individuals should:
– eat at least 1 to 1.2g/kg body weight of protein per day;
– eat MORE protein if suffering from acute or chronic illness, between 1.2-1.5g/kg body weight of protein per day;
– ingest EVEN MORE if malnourished and / or suffering from severe illness or injury, ~2g/kg body weight of protein per day;
– limit protein intake if suffering from SEVERE KIDNEY DISEASE and NOT following hemodialysis. These individuals are therefore an EXCEPTION concerning protein intake;
– use supplementation to the achieve desired protein intake levels;
– and, of course, DO the type of exercise that most effectively promotes the maintenance or increases in lean mass, which is strength training.
And no, kidney disease is NOT caused by protein intake. In a one-year follow-up study with resistance-trained men consuming ~2.51-3.32 g/kg of body weight per day for one year, there were no harmful effects on measures of blood lipids as well as liver and kidney function (Antonio et al, 2016).
Maintaining strength and lean mass (including muscle mass, bone and connective tissue mass) are identified as the first and most relevant biomarkers of health and longevity. Improving these markers produces a positive and powerful “domino effect” on most (probably all) of the other health markers. Proper protein intake and strength training is essential for building lean mass. And even more crucial as we age in order to counteract the inevitable progressive loss of efficiency in our metabolism.
Do not fear protein nor strength training. These are probably two of your best allies to live better and longer.
Don’t forget the protein at your next meal!
Nuno Correia
References:
Bauer, J. et al., 2013. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. Journal of the American Medical Directors Association, 14(8), pp.542–559.
Antonio, J., Ellerbroek, A., Silver, T., Vargas, L., Tamayo, A., Buehn, R., & Peacock, C. A. (2016). A High Protein Diet Has No Harmful Effects: A One-Year Crossover Study in Resistance-Trained Males. Journal of nutrition and metabolism, 2016, 9104792.
In previous articles (check Part 1 and Part 2) we discussed the fact that while all golfers will do everything possible to change their game (buying new clubs and new balls), many overlook one of the most decisive factors for their physical and mental success – their nutrition; we have identified some of the main reasons why golfers never change and, finally, we have pointed out five important links between golf and proper nutrition. In this article, I will share with you my concerns about the so called “normal diet” and the five most important habits you should create to start nourishing your body better.
The Western Diet
The western diet is generally used to describe popular eating habits in the USA. As these eating habits are still very popular in Europe and other parts of the non-Western world, it will probably be necessary to find a new name for this type of “diet” in the coming years (perhaps the killer diet is a good name!).
But what is the “western diet?” Well, this “diet” can be briefly characterized by a high intake of processed foods, refined carbohydrates, sugar and hydrogenated fats; and a low intake of healthy fruits, vegetables and fats. It is this “western diet” that has been associated with most western diseases or diseases of civilization (examples: cardiovascular disease, obesity, diabetes, cancer, Alzheimer, Parkinson). Interestingly, this diet may also be responsible for increased violence and decreased cognitive test scores in school-age children (attention deficit disorder) and adults. Finally, this “western diet” has also been linked to bone loss (osteoporosis) and muscle wasting (sarcopenia) in adult individuals.
Let’s look at an example of a “normal athlete” daily menu before working with me.
Breakfast: Bowl of cereals and milk + Natural orange juice + Coffee with sugar
Snack 1: Mixed toast + Chocolate milk
Lunch: Chicken salad with mayonnaise and ketchup sauce + soda + ice cream
Snack 2: Slice of cake + Juice
Dinner: Grilled fish with vegetables + Baked potatoes + Glass of red wine + Dessert
For most people, this may even be considered a good day (and indeed there are some good things in this menu), but in the long run this type of eating will greatly compromise your health and performance. For this reason, we have to discuss this situation with the person and meet their real physiological and functional needs.
The Habits you must create
Before changing their diet, it is important that people start with a firm and solid foundation, and then make the necessary changes to optimize their health and performance. Most people (athletes included) want to start building a house from the roof down or building walls without paying attention to the quality of the soil. Any of these habits are simple enough, but just like any habit you want to create, it takes time to practice to assimilate the foundations of proper nutrition.
Habit 1 – Drink more water
Sometimes we talk about so much about food and forget about water, the most important element our bodies need to function properly. It is water that regulates all the functions of our body, including the activity of all solutes (the solid materials) dissolved in it. It is water that will actually allow the nutrients we consume to reach our cells and our organs. Aim to consume 1-2 glasses of water after waking up and to drink about 2 liters / day.
Habit 2 – Eat a protein source at every meal
Eating protein at every meal is critical. Of course, some “experts” will make you believe otherwise, suggesting that protein is somehow harmful to the kidneys, unnecessary, etc. However, I want to highlight the fact that what I am looking for my clients / athletes is the best of these three worlds: better health, better body and better performance – it will be very difficult to achieve these three with a suboptimal protein intake. Some examples include: eggs, meat, fish, chicken, turkey and shellfish.
Habit 3 – Eat vegetables at every meal
Your parents and / or grandparents are right – you need to eat vegetables to prevent disease! Evidence has shown that in addition to the micronutrients (vitamins and minerals) contained in vegetables, there are also phytochemicals that are essential for optimal physiological functioning. Vegetables will help improve the acid-base balance in the body, so my advice is eating all the vegetables you can!
Habit 4 – Eat a healthy fat sources at every meal
Fat (such as protein) is an essential macronutrient and so we have to ensure proper intake every day through our diet. There has been a lot of misconception about the effects of fat on diet but the truth is that fat can be our best friend when the goal is to lose fat mass, optimize the body’s physiological response and our health. Some examples of healthy fats include: fatty fish, nuts, seeds, extra virgin olive oil, coconut and avocado.
Habit 5 – Eat more vegetables with main meals and leave the other carbohydrates to the post workout window
Another way of saying this is: if you have fat to lose, you have to earn the right to eat carbohydrates by doing vigorous exercise in the first place! Do you want to keep eating bread, pasta, rice, potatoes, crackers, sugary foods, pizzas, sweets, etc.? You can even eat them – just make sure you do it in the post workout window (1-2 hours after training) because this is the time when your body is most sensitive to insulin. As for the quality of carbohydrates, you will have more benefits if you choose gluten-free foods (rice, quinoa, chestnuts, sweet potatoes, yams, potatoes) than highly processed foods or gluten-free junk foods. Also, don’t use exercise as an excuse to treat your body like the garbage truck!
Good strokes!
Pedro Correia
All golfers have one thing in common: they will invest a lot of time and money to improve their game! They will hire the best coaches to perfect your golf swing. They will buy the best equipment available to get more accuracy and to gain distance. Those who have already realized the importance of fitness in this sport will hire the best professionals to help them gain mobility, become stronger and more resilient. Some will turn to sports psychologists to learn how to maintain a good attitude in the field, focus and concentration. They do all this in order to make more birdies. But aren’t they forgetting something?
In fact, there is something that most golfers forget to change: their low-quality diet. Most golfers have no idea how diet can influence performance. For starters, they forget that eating poorly will compromise increased muscle strength and will lead to body fat accumulation. And if you still think that having weak muscles and excess body fat is the way to go to become a top-level golfer, it’s not worth reading any further. This article is not for you.
In addition to the deleterious effects on body composition and muscle strength it must be borne in mind that eating poorly will limit focus and concentration, will lead to mood swings and may lead to poor immune function, increased chronic inflammation and general health problems. Try playing several rounds for days at a time when you are in a bad mood, in bad shape, and unable to concentrate. Poor nutrition will have a direct impact on training and competition. Lack of focus, concentration and early tiredness, especially during training and / or competitions, are signs that your nutrition is compromising your performance.
No doubt, you can be very skillful with your golf clubs, do some Ballesteros shots and do some exceptional rounds from time to time, but if you’re looking for consistency in results and longevity as a golfer, there’s no better substitute than the foods you eat every day. None!
So why do golfers never change?
There are several reasons why golfers never change and prefer to continue eating the club sandwiches, the chips and the pasta served in every clubhouse menu in the world. Let’s go through some of those reasons.
1) The myth of the pre-competition meal.
Many golfers (especially beginners) make the mistake of thinking that it is only the food eaten before and during practice and competition that can affect their game. While this may make sense at a first glance, this is not physiologically what happens – most of the energy you are using today depends on the nutrients you have eaten (and absorbed) in the last 72 hours!
Please note, I am not saying that what you eat before and after practice / competition makes no difference. Of course it does! However, these meals are not necessarily the most important. Each meal will have a certain impact on your body regardless of the time frame in which it is consumed. It is the cumulative effect of these meals that will lead to an improvement (or decrease) in performance.
In other words, in golf, there is no pre-competition or intra-competition magic meal. If you’ve waited until the day before a big competition to start eating well, it’s probably too late. I am sorry to inform you but there are no miraculous solutions, you really need to eat consistently well!
2) Golfers cannot see the link between nutrition and performance.
Most golfers do not realize the connection between nutrition and performance. And that’s because they are not aware of how diet will influence their muscle function and brain biochemistry.
In the case of golf, as this is a sport that does not involve too much energy expenditure and does not require athletes to maintain a certain body weight (as with wrestlers, gymnasts, swimmers, endurance athletes or athletes from other sports), it is thought that the need to eat well is not that important. In fact, this relationship might not seem so obvious.
But that’s not the point, golfers don’t need to be muscular and low body fat individuals as you see in fitness magazines. What they need is to develop their athletic potential so that this is not a factor that could hinder their performance on the golf course. Golf is not a very energy-intensive sport so a golfer’s nutritional needs cannot match those of a fighter or an endurance athlete. But different does not mean less important – as we saw above it is not just body composition that will be influenced by diet.
Although the link between golf and nutrition is a little less visible than in other sports, it exists. Maintaining and / or increasing muscle mass, mobility, reducing inflammation of muscles and joints, maintaining focus and concentration, preventing injuries, can all be influenced by nutrition. But only those who put the best nutritional strategies into practice will have competitive advantages (obviously this does not include Big Macs!).
3) The difficulty in changing eating habits.
Probably the most difficult hurdle for most people is overcoming the eating habits they have. Our eating patterns have been around for a long time and few people are willing to change them if there is not a strong reason (as in the case of a serious health problem). And why does this happen? Because we have not been taught to think that food is information for our cells. We have not been taught to think that food will influence the expression of our genes. We have not been taught to think that it is the everyday foods we consume from our environment that can trigger health improvement or health deterioration.
Of course there are many other reasons why golfers are reluctant to change their eating habits and why you don’t change yours. And that’s why in the next article I will continue to address this topic so you can understand why you need to change your eating habits quickly to live healthier and to take your game to the next level.
Good strokes!
Pedro Correia
Check Part 1 HERE and Part 2 HERE
Implications and practical applications of calorie restriction and intermittent fasting
And now … can the practice of calorie restriction (CR) or fasting have therapeutic value in humans? As noted earlier (Part 1), randomized controlled trials in humans studying the effects of CR and fasting in humans are scarcer. It is not easy to find volunteers to subject themselves to the “discomfort” of eating less food.
Just an aside …
Changing something in someone’s diet can be a daunting task! In fact, and based on my experience, people are generally highly resistant to changing whatever their eating patterns are and tend to defend them tooth and nail! They elaborate the most varied rationales (and very sophisticated sometimes … like the typical “but my grandfather is 90 years old and always ate this and that”) to justify the intake of certain foods that, essentially, they are just used to or like eating. Nutrition is like a religion for some, believe me …!
Back to human studies …
The other practical reason for the lack of controlled randomized human trials on the effects of CR or fasting to study its effects on life expectancy and the incidence of “age-related diseases” is that human life expectancy is long. However, some randomized controlled trials in humans point to clear benefits of CR practice in certain populations. Listed below are some of these studies, type of intervention and significant effects observed.
• Wang et al. (2013)
- Sample: obese individuals.
- Intervention: 5 days of 30% CR (low-fat / high-carb or high-fat / low-carb) after isocaloric diet period.
- Significant results:
- CR diets decreased fasting insulin and leptin levels by increasing free fatty acid levels (indicating mobilization of fat stores);
- Insulin sensitivity did not improve significantly (perhaps due to the short 5-day period), however muscle insulin signaling (in response to insulin) increased only in the low-fat / high-carb diet subjects. Note that this effect on insulin signaling in response to the low-fat / high-carb diet (rather than the high-fat / low-carb diet) may represent only a transient adaptive response due to the higher glycemic load in the diet. The short duration of the study does not allow the conclusion of a sustained improvement in insulin regulation.
- Kitzman et al. (2016)
- Sample: elderly obese individuals (67 ± 5 years) with heart failure.
- Intervention: 20 weeks of CR (350-400kcal / day deficit) with or without exercise (1 hour walking 3 days per week).
- Significant results:
- Both CR and exercise (separately) increased aerobic capacity (indicated by increase in VO2 peak), with even greater effects if combined;
- Both CR and exercise (separately) improved body composition (fat loss) with even greater effects if combined;
- CR (but not exercise) reduced the inflammatory marker C-reactive protein (CRP) and correlated with weight loss.
- Snel et al. (2012)
- Sample: obese individuals with type 2 diabetes mellitus (T2DM) and insulin-dependent.
- Intervention: 16 weeks of CR (450kcal / day) with or without exercise (1 hour + 4 30-minute sessions on a cycle ergometer per week).
- Significant results:
- Both CR and exercise improved fasting glucose, insulin and glycosylated hemoglobin (HbA1c) levels;
- The RC + exercise group lost more fat and waist circumference compared to the CR group only;
- Both CR and exercise increased insulin receptor expression and signaling (revealed by muscle biopsy) as well as peripheral insulin sensitivity.
- Pedersen et al. (2015)
- Sample: overweight or obese, non-diabetic individuals with coronary artery disease.
- Intervention: 12 weeks of CR (800-1000kcal / day) with or without exercise (3 days / week interval aerobic sled).
- Significant results:
- Separately, CR was superior to exercise in weight loss, fat mass and waist circumference, as well as fasting blood glucose, insulin sensitivity and glucose tolerance. However, CR led to better results combined with the exercise program.
- Razny et al. (2015)
- Sample: non-diabetic obese individuals
Intervention: 3 months CR (1200-1500 kcal / day) with or without 1.8 g / day omega-3 fatty acids (in a 5: 1 DHA / EPA ratio).
- Significant results:
- CR with or without omega-3 supplementation resulted in similar decrease in body weight and fat mass;
- CR had a superior positive effect on triglyceride and insulin levels when combined with omega-3 supplementation;
- Sample: non-diabetic obese individuals
RC + omega-3 (but not only CR) improved indicators of insulin resistance (HOMA index).
- Prehn et al. (2016)
- Sample: postmenopausal obese women.
- Intervention: 12 weeks CR (<800kcal / day) followed by 4 weeks on an isocaloric diet or 16 weeks on an isocaloric diet (control group). Recommendation to increase physical activity per week.
- Significant results:
- CR (but not the isocaloric diet) resulted in better scores on memory performance tests;
CR (but not the isocaloric diet) resulted in improved glycemic control and HbA1c levels;
- CR-induced increase in cerebral gray matter density was negatively correlated with glucose levels.
- CR (but not the isocaloric diet) resulted in better scores on memory performance tests;
Recommendations and Conclusions
In fact, CR or fasting interventions do indeed appear to have clear therapeutic utility in improving health parameters related to obesity, inflammation, insulin resistance, oxidative stress, and cardiac function. It is important to note that just reducing the amount of food you eat may not be enough and perhaps not recommended. Such a simplistic and long-term intervention can result in nutritional deficits and thereby boycott putative positive health outcomes. It is therefore important to monitor and ensure adequate nutrient levels through supplementation and / or in choosing nutritionally dense foods. Also noteworthy are the positive synergistic effects that CR seems to have when combined with exercise (Snel et al., 2012; de Luis et al., 2015; Kitzman et al., 2016), which may be prescribed concomitantly. In this context, a very mild CR intervention (e.g. 10% deficit) or short intermittent fasting periods (> 14 hours and does not need to be daily) combined with exercise may have very positive effects and are likely to have higher compliance compered to more aggressive fasting or CR interventions.
Of course, severe and prolonged CR with no exercise (especially strength training) can induce lean mass loss that is highly undesirable if the goal is to improve health. Once again, and as with almost everything, the secret lies in the right dosage! In certain populations such as pregnant women (or women trying to conceive) and young growing individuals, prolonged CR interventions should be avoided as they may compromise development. However, I emphasize again that the most important thing is not to ingest “calories” but ingest “nutrients”! In older individuals with sarcopenia, CR should perhaps be avoided, although the most determining factors for reversing sarcopenia are strength training and adequate protein intake (which should be higher for older individuals (> 2g / kg bodyweight).
Regarding the specific case of intermittent fasting (note: the focus of this article is not to discuss the use of intermittent fasting as a fat loss and / or maintenance strategy or muscle mass gains in the sports context, but rather its potential for general health benefits) although few, the available randomized controlled trials in humans indicate that intermittent fasting do offer beneficial effects similar to those of constant CR and perhaps is easier to implement (Donati et al., 2008; Marzetti et al., 2009; Alirezaei et al., 2010; Arum et al.; 2014; Godar et al., 2015). Irregular fasting episodes (e.g. not eating breakfast once or twice a week on non-training days) can not only be a strategy easy to implement strategy as a mean to control total weekly calories ingested, and it also has positive hormonal effects is mediated by some of the mechanisms described above. “Hormesis” is defined as a mild stressor that is beneficial to health, stress resistance, growth and longevity. It results from exposure to an “adequate” dose of a stressor. CR or fasting (or exercise) is something that we are evolutionarily designed to tolerate, and which at the right dose gives us benefits and greater resilience. The occasional stress of not eating can be a “healthy discomfort”!
Until next time!
Nuno Correia
What mechanisms underlie the effects of calorie restriction or intermittent fasting on longevity and “age-related diseases”?
But after all what is “getting old” … and why …?
Aging has been characterized by several authors as a process of progressive deterioration of molecular, cellular and tissue structures and functions that is conditioned by genetic and environmental factors (Hu & Liu, 2014). This multifactorial and complex process resulting from this progressive loss of function makes the individual more vulnerable to disease and ultimately leads to death. The main determinants (resulting from genetic predisposition and environmental factors) that characterize the aging process at the cellular level have been identified as: free radical damage; mitochondrial dysfunction results in an accumulation of reactive oxygen species (ROS) and consequent oxidative stress; decrease and inefficiency of autophagy (an evolutionarily conserved process of recycling and “cellular waste removal” that is essential for cellular integrity, more details a bit ahead); changes in hormone-related signaling processes such as type 1 insulin-like growth factor (IGF-1), insulin and growth hormone; change in cholesterol and glucose metabolism; telomere shortening (Testa et al., 2014).
Now it seems that the aging process is indeed multifactorial. Probably the various aging theories (see part 1) are correct! In general, molecular processes are becoming more inefficient, slower and the system is progressively moving towards entropy. However, it seems that knowing the autophagy process (whose decline is associated with aging) may offer a “new” perspective on aging. Autophagy (or “self-digestion”) has been defined as an evolutionarily conserved (normal and important) catabolic process characterized by degradation in the lysosomes (cell organelle that functions as a “litter”) of damaged organelles, “defective” proteins and intracellular pathogens (Lavallard et al., 2012). Autophagy provides macromolecule degradation and recycling, not only providing new nutrients and energy during energy restriction (during calorie restriction or fasting), but also preventing the accumulation of cell metabolism by-products and protein aggregates in the cytoplasm. Therefore, autophagy is a protective and essential process for cellular homeostasis (Rubinsztein, Mariño & Kroemer, 2011) (note: rest assured that autophagy will “eat all the muscles” for a few hours without eating. That simply does not happen!). In fact, several authors have pointed deficient autophagic capacity as an important mediator of cellular senescence and consequent occurrence of “diseases or characteristics of old age” such as cardiovascular and neurodegenerative diseases; oxidative stress; weak immune system; chronic inflammation; osteoporosis; sarcopenia; diabetes; obesity; cancer (Pallauf & Rimbach, 2013; Pyo, Yoo & Jung, 2013). Specifically, reviews of mechanistic animal studies have indicated that loss of function in autophagy-related genes has resulted in intracellular accumulation of defective proteins and organelles and consequently in the acceleration of aging, while promoting autophagic activity increased life expectancy (Yen & Klionsky 2008)
(Note: Autophagy mechanisms have been in the mainstream news since 2016 with the Nobel Prize in Medicine 2016 awarded to the Japanese biologist Yoshinori Ohsumi. Their findings in autophagy mechanisms point in the direction that this cellular cleaning and recycling process is essential to prevent neurodegenerative and other diseases. This is the LINK for the news article).
Overall, and since the first rat studies by Dr. Clive McCay in 1935, calorie restriction has been extensively reviewed and recognized as a “potent” anti-aging strategy! Interventions in various types of animal species (from invertebrates to larger mammals such as primates) have shown that calorie restriction (without malnutrition) not only increases life expectancy (average and maximum), but delays the onset of so-called “age-related diseases ”(Martin, Mattson & Maudsley, 2006; Xiang & He, 2011; Lee & Min, 2013; Kitada & Koya, 2013b; Szafranski & Mekhail, 2014; Testa et al., 2014). The intermittent fasting regime (nothing more than another calorie restriction strategy as described in part 1 of this article) seems to offer the same kind of benefits (Martin, Mattson, & Maudsley, 2006; Robertson & Mitchell 2013).
Now the mechanisms by which calorie restriction or fasting induce health benefits appear to be (to a large extent) related to this antagonistic relationship between insulin signaling and autophagy. It is easy to understand, being autophagy a catabolic process (essential, normal and protective, I highlight that again) and the activation of insulin signaling pathways an anabolic process (equally important and essential in protein synthesis, insulin is not the “villain”), when one of these pathways is activated the other will be inhibited. Practically speaking, fasting activates the autophagy “machinery” and eating a meal (mainly containing protein and / or carbohydrates) activates the insulin signaling “machinery”. What seems to be essential is in fact that there are periods that allow the process of elimination and recycling provided by autophagy, and for this to happen it is necessary not to eat for a while. If there is no “room” for this process, due to constant food intake, it may lead to constant and “aberrant” insulin signaling state which may lead to many diseases that are usually associated with poor glucose and insulin metabolism which make the most of the so-called “age diseases”.
(Warning: the “less nerds“ should skip next paragraph)
Briefly, some of the mechanisms identified in animal studies that appear to underlie the health benefits induced by caloric restriction or intermittent fasting through regulation of autophagy and insulin pathway signaling are: 1) Inhibition of insulin / IGF-1signaling (due to the decrease in circulating amino acids and glucose) and its target pathways protein kinase B (PKB) / mammalian target of rapamycin (mTOR) ; 2) Activation of the sirtuin 1 pathway (SIRT1) due to the increase in NAD + / NADH ratio, which targets include activation of adenosine monophosphate protein kinase (AMPK), forkhead box O (FOXO) transcription factors, proliferator- activated gamma receptor-1-alpha coactivator (PGC-1α) (a mitochondrial biogenesis factor), and inhibition of the pro-inflammatory transcription factor NFkB; 3) Activation of the AMPK pathway due to the intracellular increase of the AMP / ATP ratio, which in turn induces up-regulation of FOXO and PGC-1α transcription factors and inhibition of the PKB / mTOR pathway. (Martin, Mattson & Maudsley, 2006; Han & Ren 2010; Rubinsztein, Mariño & Kroemer, 2011; Yen & Klionsky, 2008; Xiang & He, 2011; Pallauf, & Rimbach, 2013; Pyo, Yoo, & Jung, 2013; Hu & Liu, 2014; Szafranski & Mekhail, 2014; Amigo & Kowaltowski, 2014; Testa, G. et al., 2014; Madeo et al., 2015).
In humans, despite the smaller abundance of randomized controlled trials (for the reasons mentioned in Part 1 of this article), several reviews of intervention and observational studies (Yen & Klionsky, 2008; Marzetti, E. et al., 2009; Han & Ren 2010; Robertson & Mitchell, 2013; Testa et al., 2013; Madeo et al., 2015; Fan et al., 2016) indicate that the putative health benefits induced by calorie restriction or intermittent fasting are based on the same mechanisms related to insulin pathway signaling and regulation of autophagy. Some pointed benefits include: longer healthy longevity; better lipid profile; controlled blood pressure; optimization of diastolic and systolic function; better homeostatic control of insulin and glucose; better sensitivity to insulin and glucose; lower incidence of neurodegenerative diseases; lower adiposity; better mitochondrial biogenesis in the skeletal muscle; higher antioxidant capacity; lower levels of ROS and oxidative stress.
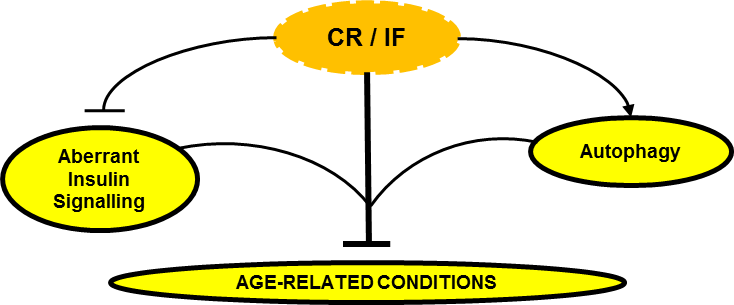
Can Calorie Restriction / Intermittent Fasting (CR / IF) alleviate age-related disease by regulating “aberrant” insulin signaling and autophagy?
In conclusion, the effect of caloric restriction or intermittent fasting on the regulation of insulin signaling and autophagy seems to emerge as a central regulatory axis that deserves attention (at least from me).
In the third part of this article I will then discuss what may be practical implications and applications of calorie restriction or fasting. Should we all do calorie restriction? Permanently? For how long? How much? What stage of life? Under what health conditions? Is the “intermittency” factor that delivers the best benefits?
Stay around!
Nuno Correia
References:
Amigo, I. & Kowaltowski, A.J., 2014. Dietary restriction in cerebral bioenergetics and redox state. Redox Biology, 2(1), pp.293–304.
Dröge W., 2009. Avoiding the First Cause of Death. New York, Bloomington. iUniverse, Inc.
Fan, J. et al., 2016. Autophagy as a Potential Target for Sarcopenia. Journal of Cellular Physiology, 231(7), pp.1450–1459. [Epub 2015 Dec 10].
Han, X. & Ren, J., 2010. Caloric restriction and heart function: is there a sensible link? Acta pharmacologica Sinica, 31(9), pp.1111–1117.
Hu, F. & Liu, F., 2014. Targeting tissue-specific metabolic signaling pathways in aging: the promise and limitations. Protein & cell, 5(1), pp.21–35.
Lavallard, V.J. et al., 2012. Autophagy, signaling and obesity. Pharmacological Research, 66(6), pp.513–525.
Lee, S.-H. & Min, K.-J., 2013. Caloric restriction and its mimetics. BMB reports, 46(4), pp.181–7.
Lee, S.-H. & Min, K.-J., 2013. Caloric restriction and its mimetics. BMB reports, 46(4), pp.181–7.
Lindeberg, S., 2010. Food and Western Disease: Health and Nutrition from an Evolutionary Perspective. Oxford, United Kingdom: Wiley-Blackwell.
Madeo, F. et al., 2015. Essential role for autophagy in life span extension. Journal of Clinical Investigation, 125(1), pp.85–93.
Martin, B., Mattson, M.P. & Maudsley, S., 2006. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing research reviews, 5(3), pp.332–53.
Masoro.E. L., 2002. Caloric Restriction: A Key to Understanding and Modulating Aging. Texas, USA: ELSEVIER.
Pyo, J.O., Yoo, S.M. & Jung, Y.K., 2013. The interplay between autophagy and aging. Diabetes and Metabolism Journal, 37(5), pp.333–339.
Robertson, L.T. & Mitchell, J.R., 2013. Benefits of short-term dietary restriction in mammals. Experimental gerontology, 48(10), pp.1043–8.
Rubinsztein, D.C., Mariño, G. & Kroemer, G., 2011. Autophagy and aging. Cell, 146(5), pp.682–695.
Rubinsztein, D.C., Mariño, G. & Kroemer, G., 2011. Autophagy and aging. Cell, 146(5), pp.682–695.
Szafranski, K. & Mekhail, K., 2014. The fine line between lifespan extension and shortening in response to caloric restriction. Nucleus, 5(1), pp.56–65.
Testa, G. et al., 2014. Calorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevity. Current pharmaceutical design, 20(18), pp.2950–77.
Xiang, L. & He, G., 2011. Caloric restriction and antiaging effects. Annals of Nutrition and Metabolism, 58(1), pp.42–48.
Yen, W.-L. & Klionsky, D.J., 2008. How to live long and prosper: autophagy, mitochondria, and aging. Physiology (Bethesda, Md.), 23(70), pp.248–262.
CAN CALORIE RESTRICTION OR INTERMITTENT FASTING HELP PREVENT “AGE-RELATED DISEASES” AND LIVE LONGER? – PART 1
Introduction
In this article I will look into the possibility of calorie restriction or intermittent fasting, which is just an alternative strategy to induce a caloric deficit, effective nutritional therapeutic strategies to prevent, alleviate or even eliminate some of the so-called “age-related diseases”, thus contributing for a better and longer life. It is important to note that most studies on the effects of calorie restriction or intermittent fasting (or intermittent calorie restriction) on life expectancy are mechanistic, and conducted in animal and / or in vitro models. It is understandable that there are fewer human intervention studies in this area. If we think about it, it is not easy to conduct studies with humans on calorie restriction to study its effects on life expectancy and the incidence of “age-related diseases”. Not only it is not easy to recruit people to voluntarily incur in a calorie restriction period, it is also not practical to study in humans (in a randomized controlled manner) the effects of calorie restriction or fasting on life expectancy, because they simply “live a long time”. ” In order to obtain timely results, it is essential to conduct studies on species with a shorter life expectancy. However, observational studies and some human intervention studies (discussed later) appear to confirm the same health benefits and molecular mechanisms as those observed in animals.
It should also be noted that, in the experimental context, caloric restriction is defined as “reduced food intake without malnutrition”. In other words, nutritional interventions imply a 10-40% reduction in daily caloric requirements in which only calories, and not nutrients, are restricted (in most controlled studies this is ensured with vitamin and mineral supplementation) (Kitada & Koya, 2013b; Robertson & Mitchell, 2013). This notion is important! Caloric deficit does not imply nutrient deficit and caloric surplus does not imply that nutrient requirements are met. Intermittent fasting will be no more than an alternative method of calorie restriction in which food intake is restricted for a certain period of time (usually 16 to 24 hours) followed by an unrestricted intake period and has been touted as producing beneficial health effects similar to more constant calorie restriction protocols (Martin, Mattson, & Maudsley, 2006; Robertson & Mitchell 2013).
Part 1
Should we accept being “sick” just because we get older?
It is recurrent to hear that the disease is something that “comes with the age package.” In fact, getting older is a drag! The general perception of a progressive decline of all our capabilities as we age is, unfortunately, not an illusion. There are several theories about aging. While certainly a very interesting topic, a detailed description of the various theories of aging is not the purpose of this article. These are some of mechanisms underlying the aging process which are generally pointed out as the main ones:
- The “Hayflick limit” (phenomenon discovered by Leonard Hayflick) determines that human cells have a replication limit number, after which they become senescent. Telomeres (i.e. a kind of protective “helmets” at the end of each chromosome) become progressively shorter with each cell division (Shay & Wright 2000). However, DNA methylation (an essential and repairing process consisting of the addition of methyl groups to DNA and which can be promoted by the abundance of dietary methyl donors for example) is said to be protective of telomere length and that way to postpone cell death and aging. For example, in animal models, hypomethylation of the enzyme telomerase reverse trancriptase has led to the preservation of leukocyte telomere length (Zhang et al. 2003; 2014). In this example, it is plausible to infer that delaying leukocyte senescence (through methylation and consequent telomere length conservation) may contribute to a stronger immune system and thus positively influence longevity.
- This theory suggests that unresolved chronic inflammation induces the human organism not to allocate resources for the functioning of other body functions (as they are permanently allocated to unresolved inflammation) and thus leading to early aging of various organs and tissues, and the early onset of “age-related diseases”.
- This theory, originally proposed by Dr. Denham Harman in 1956, is based on the premise that the aging process is mediated by free radical damage. Theoretically, by reducing free radical accumulation (e.g. reactive oxygen species) and at the same time increasing the antioxidant capacity of the organism (increasing glutathione, and antioxidant enzymes such as SOD and catalase), tissue damage can be prevented (by slowing down aging process) and to prevent the occurrence of “age-related diseases” and consequently contribute to increase functional longevity (Harman, 1988; 2006).
Very well, getting older is inevitable! We already know that. However, if we give it a little thought, all the mechanisms mentioned have an environmental root, that is, we can, to some extent, control them through decisions that we make every day. Namely decisions about what we eat and how we move. And this is good news! It is in fact in our hands to slow down the process of senescence and prevent the onset of the so-called ‘’age-related diseases’’. Note that if for us (Westernized world) it is statistically “normal” to grow old with diabetes, hypertension, cancer, dementia, sarcopenia, osteoporosis, cardiovascular disease, insulin resistance, obesity and chronic inflammation (because the population studied incurs in a lifestyle that leads to disease), in other contemporary (non-Westernized) populations such diseases are rare or even non-existent. In this context, I invite the reader to consult what I consider to be one of the best books I know about nutrition and lifestyle, and its relation to the incidence of so-called “Western” diseases: Food and Western Disease: Health and Nutrition from an Evolutionary Perspective by Staffan Lindeberg. In fact, if we want to aim at maximizing health and lifespan potential, we should not just look at what is ‘’normal’’ in a given population, because that may be a sick population. Rather, we should look for what is “biologically normal” for a human being! A species that is designed (evolutionarily) to deal with a range of environmental stimuli that include certain levels of physical activity, nutrition, sun exposure and sleep. And, although aging is a normal process, it should not be “biologically normal” to age with chronic disease.
In this context, the Okinawa Centenarian Study is also frequently cited. The Okinawan population has the highest ratio of (healthy) centenarians on the planet (50 / 100,000 vs 10-20 / 100,000 in the USA) and as such is of greatest interest to study the factors that potentiate this kind of longevity. One of the factors identified (in addition to an appreciable level of physical activity and social interaction) was the fact that populations over 70 eat about 11% of calories below (about 1785kcal / day, which is a very moderate level of calorie restriction) than would be recommended for maintaining body weight (according to the Harris-Benedict equation), however on a nutrient-rich diet (Wilcox et al., 2006).

What we can do to live longer and better is one of my main interests. As I mentioned, our choices regarding the type of exercise, food we eat and other lifestyle factors can affect how long we live and, perhaps most importantly, how healthy and functional we live. In Part 2 of this article I will discuss some mechanisms by which nutritional interventions such as calorie restriction or intermittent fasting can lead to health benefits. And in Part 3 I will address the possible implications and practical applications of the practice of calorie restriction or fasting, as well as which populations can benefit most from these nutritional strategies and whoshould avoid them.
Stay around!
Nuno Correia
References
Dröge W., 2009. Avoiding the First Cause of Death. New York, Bloomington. iUniverse, Inc.
Harman D., 1988. Free radicals in aging. Mol Cell Biochem. Dec; 84(2), pp.155-161.
Harman D., 2006. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. May;1067, pp.10-21.
Kitada, M. & Koya, D., 2013b. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes & metabolism journal, 37(5), pp.315–25.
Lindeberg, S., 2010. Food and Western Disease: Health and Nutrition from an Evolutionary Perspective. Oxford, United Kingdom: Wiley-Blackwell.
Martin, B., Mattson, M.P. & Maudsley, S., 2006. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing research reviews, 5(3), pp.332–53.
Masoro.E. L., 2002. Caloric Restriction: A Key to Understanding and Modulating Aging. Texas, USA: ELSEVIER.
Robertson, L.T. & Mitchell, J.R., 2013. Benefits of short-term dietary restriction in mammals. Experimental gerontology, 48(10), pp.1043–8.
Shay J.W., Wright W.E. 2000. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. Oct;1(1), pp.72-76.
Zhang D. et al., 2013. Homocysteine-related hTERT DNA demethylation contributes to shortened leukocyte telomere length in atherosclerosis. Atherosclerosis. Nov; 231(1), pp.173-179.
Zhang D.H., Wen X.M., Zhang L. & Cui W., 2014. DNA methylation of human telomerase reverse transcriptase associated with leukocyte telomere length shortening in hyperhomocysteinemia-type hypertension in humans and in a rat model. Circ J. 78(8), pp.1915-1923.
Wilcox D.C. et al., 2006. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology 7, pp.173–177.







